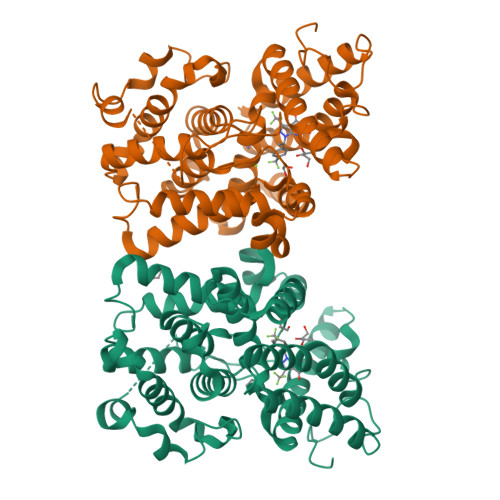
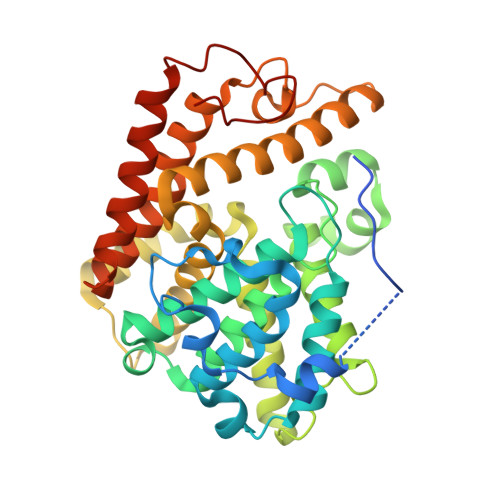
Structure-Guided Design and Procognitive Assessment of a Potent and Selective Phosphodiesterase 2A Inhibitor.
Stachel, S.J., Berger, R., Nomland, A.B., Ginnetti, A.T., Paone, D.V., Wang, D., Puri, V., Lange, H., Drott, J., Lu, J., Marcus, J., Dwyer, M.P., Suon, S., Uslaner, J.M., Smith, S.M.(2018) ACS Med Chem Lett 9: 815-820
- PubMed: 30128073
- DOI: https://doi.org/10.1021/acsmedchemlett.8b00214
- Primary Citation of Related Structures:
6CYB, 6CYC, 6CYD - PubMed Abstract:
Herein we describe the development of a series of pyrazolopyrimidinone phosphodiesterase 2A (PDE2) inhibitors using structure-guided lead identification and design. The series was derived from informed chemotype replacement based on previously identified internal leads. The initially designed compound 3 , while potent on PDE2, displayed unsatisfactory selectivity against the other PDE2 isoforms. Compound 3 was subsequently optimized for improved PDE2 activity and isoform selectivity. Insights into the origins of PDE2 selectivity are described and verified using cocrystallography. An optimized lead, 4 , demonstrated improved performance in both a rodent and a nonhuman primate cognition model.
Organizational Affiliation:
Merck & Co. Inc., P.O. Box 4, West Point, Pennsylvania 19486, United States.